Basics of Supercritical Fluid Chromatography
SFC Basics Course
1 - What are Supercritical Fluids and the History of SFC
2 - SFC Instrumentation and Advantages of SFC
3 - SFC Analytical Operating Conditions Part I Stationary Phases
4 - SFC Analytical Operating Conditions Part 2 Mobile Phases and Other Parameters
5 - Preparative SFC
Welcome
Welcome to our Free Online Course: Basic Introduction to Supercritical Fluid Chromatography (SFC)!.
We’re excited to have you with us for this five-week journey into the world of SFC. Each week, you’ll receive an email with a link designed to give you an introduction to SFC as a chromatographic technique to add to your laboratory toolbox. At the end of the whole course, there is a quiz for you to show how far your knowledge has grown and a Certificate of Completion when you pass the quiz.
In this first week, we will establish the foundations of what supercritical fluids are and the history of SFC. Let’s dive right in!
Supercritical Fluids
Supercritical fluid chromatography (SFC) is a separation technique that uses supercritical carbon dioxide as the mobile phase. Although SFC has existed for a long time, recent advancements in SFC instrumentation and column technologies have made it a more commonly used technique for regular lab use and measurements. By using supercritical carbon dioxide, which can be mixed with water-soluble solvents despite its low polarity, SFC offers potential advanced separation methods that are different from conventional gas chromatography (GC) and high performance liquid chromatography (HPLC) separations. This week's course describes what is supercritical carbon dioxide.
Substances can change their state between solid, liquid and gas depending on the temperature and pressure. Vaporisation and sublimation are phenomena that occur when heat activates the thermal motion of solid or liquid molecules in a substance in its current state. For example, solid CO2 (dry ice) in a sealed container sublimates to reach a supercritical state under high temperature and high pressure conditions (Fig. 1). A supercritical state is when substances have properties of both gas and liquid, and they are thus called supercritical fluids.
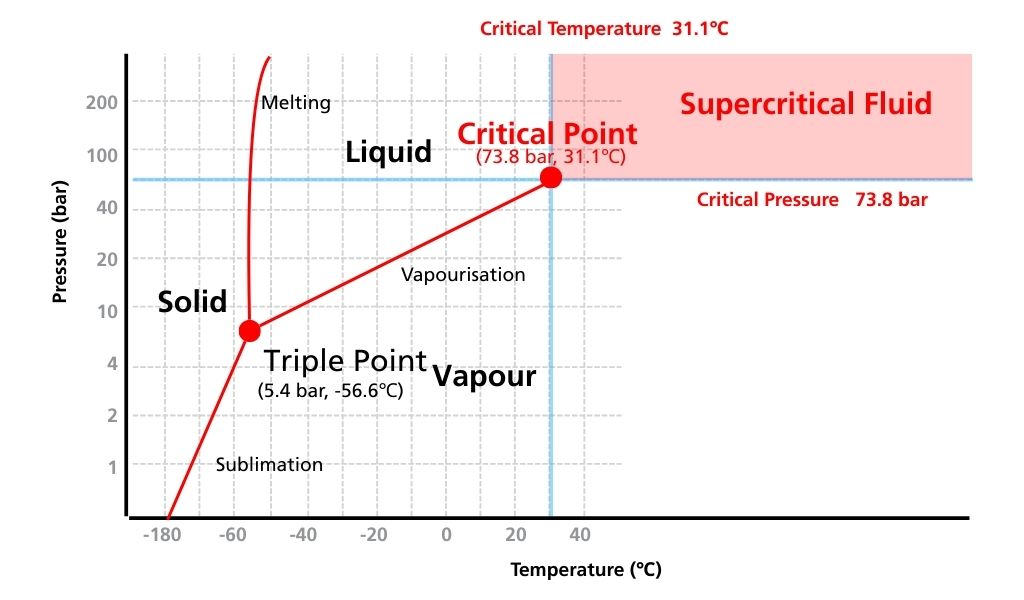
Fig. 1 Phase diagram for carbon dioxide
-
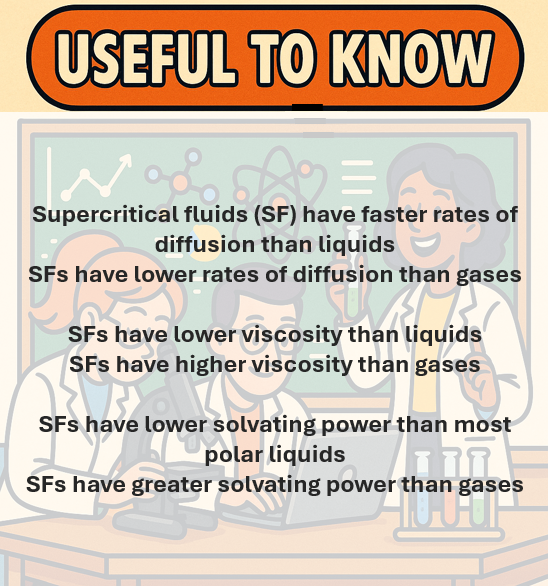
- This point in the phase diagram with corresponding conditions of temperature and pressure is called the Critical Point (Table 1). Supercritical fluids have a higher coefficient of diffusion with lower density and viscosity than liquids. Consequently, higher diffusivity results in better distribution into the mobile phase and better separation, and lower viscosity makes SFC a faster method than HPLC.
Table 1 Physical properties of supercritical fluids
| Diffusion Coefficient (m2/S) | Density (g/cm3) | Viscosity (g/cm·s) | |
|---|---|---|---|
| Liquids | 10-6 | 1 | 10-2 |
| Supercritical Fluids | 10-3 | 0.2-0.8 | 10-3 |
| Gases | 10-1 | 10-3 | 10-4 |
The critical pressures and critical temperatures for generating a variety of supercritical fluids are shown in Fig. 2. Water is commonly used for HPLC separations, but due to its critical pressure of 221 bar and critical temperature of 374 °C, it cannot be easily kept under conditions necessary for transitioning to a supercritical state. In contrast, supercritical carbon dioxide has a critical pressure of 73.8 bar and a critical temperature of 31.1 °C, hence allowing transition to the supercritical state under relatively modest conditions.
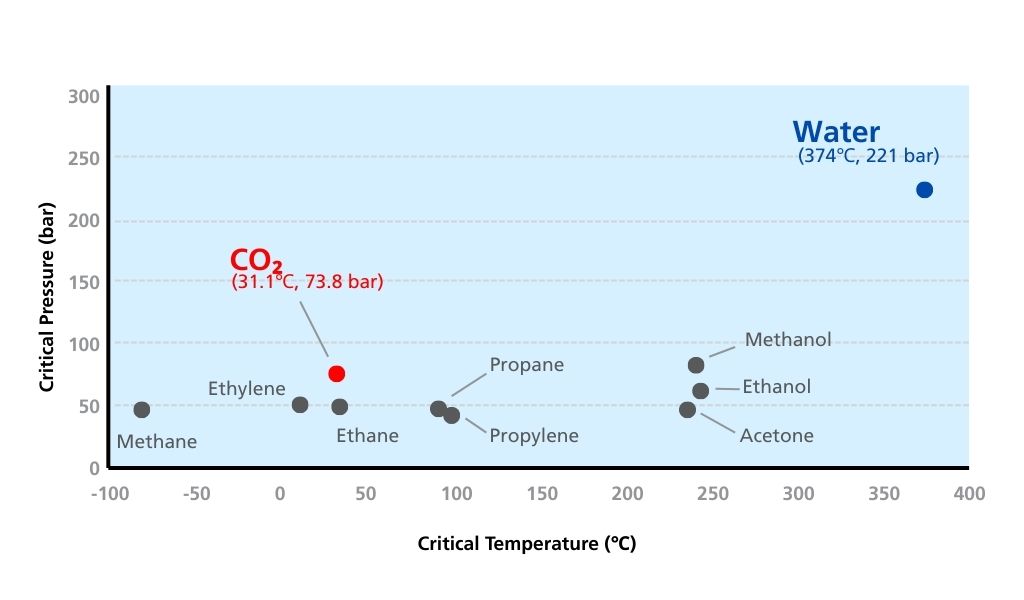
Fig. 2 Conditions for generating supercritical fluids for methane, ethylene, ethane, carbon dioxide, propane, propylene, acetone, ethanol, methanol and water

Supercritical carbon dioxide offers the following advantages:
- (1) Handling
With critical points at 31.1 °C and 73.8 bar, CO2 becomes a supercritical fluid under modest conditions.
- (2) Safety
CO2 is not flammable and has minimal risk of explosion even if used compressed. CO2 offers intrinsically low toxicity when compared to other organic solvents.
- (3) Cost
CO2 is mainly sold as a liquid stored in high-pressure cylinder and in most cases can be acquired at a low cost.
- (4) Environmental Friendliness
Other organic solvents are incinerated for disposal, which emits large amounts of energy and CO2. However, liquified CO2 used in SFC is collected as a byproduct of fermentation processes or chemical plants. That means using liquified CO2 causes minimal environmental impact
Brief History of SFC
Research on SFC as an analytical technique began in the 1960’s, where it was first reported by Klesper et al1 as high-pressure gas chromatography and was seen as an extension of GC. However, there is a clear distinction between GC and using supercritical fluids where in GC the mobile phase gas is an inert carrier whilst in what was later described as SFC, the supercritical gas mobile phase is acting as a solvent and plays a critical role in separation.
The technique did not begin to evolve and gain traction until the late 1990’s. The delay was primarily due to the increased focus on HPLC as an analytical technique at the time which was seen to be more robust and applicable. HPLC also solved many issues on retaining compounds typically unsuited to conventional GC such as polar analytes. The columns posed another issue, where the efficiency was quite poor. Over the years, there have been investigations into improving the column packing, including looking at packed columns and open tubular column formats.
In the late 1960’s, Giddings et al noticed that there was a convergence between this high-pressure gas chromatography and conventional liquid chromatography, due to the gas molecules possessing a liquid-like density which gave it interesting properties2. This drove the innovation and instrumentation which can be seen today. Nowadays, SFC refers to a chromatographic technique involving supercritical carbon dioxide as the mobile phase. Since SFC uses CO2 like a liquid, it is also referred to as “LC using CO2.”
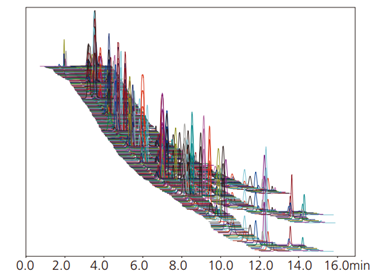
Fig. 3 Mass chromatogram of standard pesticide mixture solution
When used in conjunction with organic modifiers, such as methanol, ethanol or isopropanol, the chromatographic differences can be substantial and offer the chromatographer a good alternative in their analytical toolbox.
Before commercialisation of SFC, measuring compounds with a wide range of properties required using multiple analytical techniques; however, using a combination of SFC and LC for “unified chromatography” enables simultaneous analysis of heterogeneous components.3
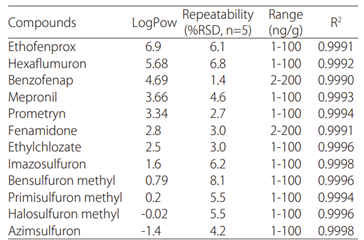
Fig. 3 shows the simultaneous analysis of 510 standard pesticides in a single injection using SFC. Table 2 shows the octanol-water partition coefficient, repeatability, and linearity results for a few target pesticides. The technique enables accurate simultaneous analysis of compounds with a wide range of polarities.
Table 2 Repeatability and linearity for pesticide analysis
We hope you have enjoyed this initial installment of the SFC basics introduction. Next week we will look into the different components which make up an SFC instrument and the advantages of SFC as an analytical technique.
Your Shimadzu Chromatography Team
References
1High pressure gas chromatography above critical temperatures,K. Klesper, A.H. Corwin, D.A. Turner, J. Org. Chem. 1962, 27, 700-701
2Dense gas chromatography at pressure to 2000 atmospheres, C.G. Giddings, M.N. Myers and J.W. King, J. Chromatogr. Sci., 1969, 7, 276-283
3Simultaneous analysis for water- and fat-soluble vitamins by a novel single chromatography technique unifying supercritical fluid chromatography and liquid chromatography, K. Taguchi, E. Fukusake, T. Bamba, J Chromatogr. A, 2014, 1362, 270-277
Mini Quiz
(1) To be supercritical do you want the pressure and temperature to be:
Above the critical point?
Below the critical point?
Exactly on the critical point?
(2) Why is supercritical CO2 advantageous over other substances? (Select all that apply)
Cheaper
Easier to reach the critical point
Minimal explosion risk
CO2 is inherently pure
Guaranteed reproducibility
(3) In what decade did Klesper et al first discuss high pressure gas chromatography?
1950’s
1960’s
1970’s
1980’s
Scroll down for the answers!
Related Resources
Answers!
(1) To be supercritical do you want the pressure and temperature to be:
Above the critical point? ü
(2) Why is supercritical CO2 advantageous over other substances? (Select all that apply)
Cheaper ü
Easier to reach the critical point ü
Minimal explosion risk ü
(3) In what decade did Klesper et al first discuss high pressure gas chromatography?
1960’s ü






