Basics of Supercritical Fluid Chromatography
SFC Basics Course
3 - SFC Analytical Operating Conditions Part I Stationary Phases
4 - SFC Analytical Operating Conditions Part 2 Mobile Phases and Other Parameters
5 - Preparative SFC
Welcome Back!
Welcome back for this third session on a brief introduction into supercritical fluid chromatography. Hopefully you are now aware of what a supercritical fluid is and how an SFC instrument needs to be adapted in comparison to an LC to work efficiently. The operating parameters can highly influence the success of the chromatographic separation, therefore, it is important to know which variables need to be investigated. In this section, we will look at the effect of stationary phase type.
The approach for the SFC analytical condition development process is basically the same as for determining analytical conditions for HPLC. HPLC can generally target any component in compounds that can be dissolved in the mobile phase. In contrast, SFC can be used to analyse any compound that is compatible with supercritical carbon dioxide and can be dissolved in an organic solvent. If chromatography is used for quantitative analysis, then optimal analytical conditions should achieve resolution of 1.5 or more. The basic equation for calculating resolution Rs is indicated below
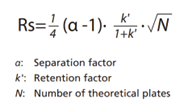
The α, k, and N values that affect Rs are all independent factors that vary depending on the column and mobile phase used, where N represents separation efficiency and α selectivity. In particular, separation can be improved by increasing N and α values. The supercritical carbon dioxide used for SFC offers different properties than mobile phase solvents used for HPLC, therefore when a column used for HPLC is used for SFC, it can sometimes result in different elution behaviour (Fig. 1).
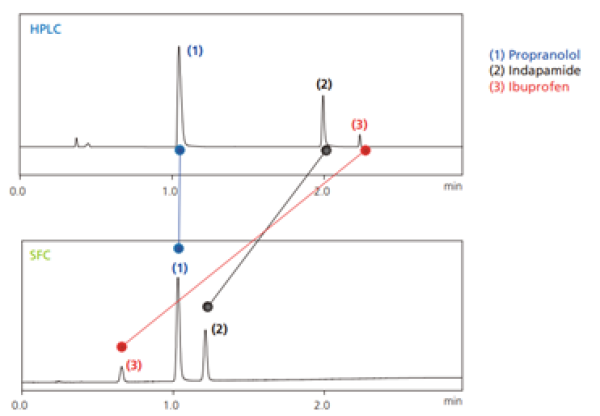
Fig. 1 Separation of three types of pharmaceutical ingredients (upper: HPLC, Lower: SFC)
SFC Stationary Phases
In principle, the various columns used for HPLC can also be used for SFC, but in order to ensure the CO2 is kept in a supercritical fluid state, make sure the column has the pressure capacity necessary for maintaining the required pressure level. SFC columns are generally compatible with fittings used in HPLC systems, but it is important to confirm the pressure capacity and other requirements for actual use with respective column manufacturers. Columns used in SFC must be able to withstand high pressures at both the inlet and outlet due to the back pressure regulators that maintain SFC conditions and keep CO2 in a supercritical state. Polymer-based columns require special care, as pressure tends to increase during use when the stationary phase expands. In addition, reducing the amount of elastomeric material in the column helps ensure reliable, leak-free performance.
Since supercritical carbon dioxide has a polarity comparable to n-hexane, the main SFC separation modes are expected to resemble those of normal-phase chromatography. However, unlike HPLC, SFC can utilize both normal phase columns (e.g., silica, diol, CN) and reversed phase columns (e.g., C18, cholesteryl, phenyl). Furthermore, the supercritical carbon dioxide used in SFC exhibits high diffusivity and low molecular density, making it more susceptible to secondary interactions than HPLC. As a result, separation behaviour can vary significantly depending on the stationary phase. Therefore, to determine optimal conditions, it is essential to evaluate a wide range of stationary phase types.
Although supercritical carbon dioxide has low polarity similar to n-hexane, as mentioned above, it can be blended with highly polar solvents. This enables normal-phase columns to be replaced with reversed-phase columns without changing the mobile phase type or modifier composition. Figure 2 shows a comparison of the elution order for HPLC and SFC. In HPLC, mobile phases must be tailored to either reversed phase or normal phase separation modes, requiring the system to be purged each time the mode is switched. In contrast, SFC allows the same mobile phase conditions to be used for both separation modes, meaning results can be assessed simply by changing the column.
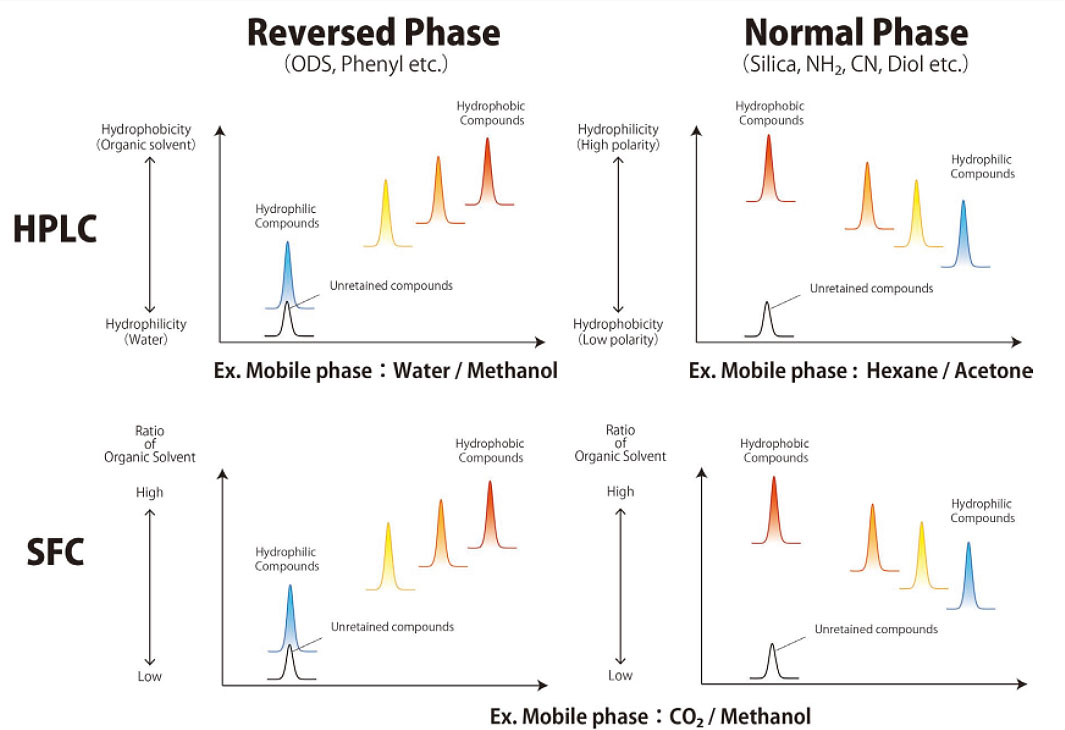
Fig. 2 Comparison of elution order for HPLC and SFC
Since normal phase separation is the primary mode used in SFC, normal phase columns such as Shimadzu’s UC-Diol and UC-Diol II are widely adopted as the first choice for SFC systems. The next most commonly used are UC-Py columns, valued for their similarity in separation behaviour to ethylpyridine-based columns. In contrast to HPLC, which requires aqueous or non-aqueous mobile phases with significantly different compositions for reversed phase and normal phase analyses respectively, SFC employs the same mobile phase – a mixture of supercritical fluid carbon dioxide and a modifier (a water-soluble organic solvent such as methanol) - regardless of the stationary phase. This means a single mobile phase composition can be applied across multiple column types, enabling seamless serial analysis. Since supercritical carbon dioxide can penetrate tiny pores more easily, it promotes higher interaction with the stationary phase. That means SFC offers the ability of separating isomers or other compounds that are difficult to be separated by HPLC. In particular, columns with unique or multiple interaction mechanisms can further enhance separation performance in SFC. For SFC, no single column offers the broad applicability comparable to the C18 column in HPLC reversed phase chromatography
This is illustrated in Fig. 3, which shows chromatograms obtained using diol group, hydroxyphenyl group, and cholesteryl group columns on an acidic, basic and neutral compounds (ibuprofen, propranolol and indapamide, respectively).
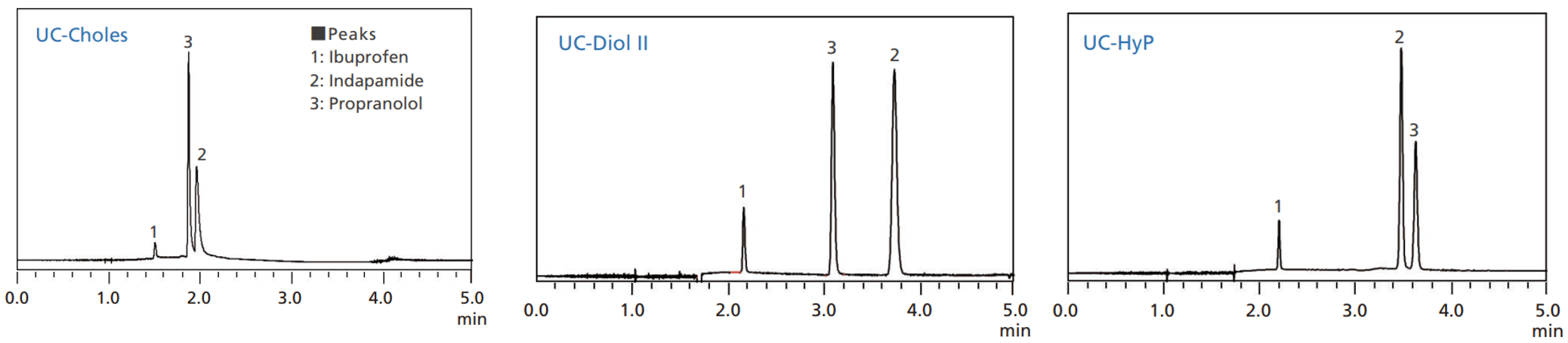
Fig. 3 Differences in retention behaviour for different stationary phases (normal phase for diol, normal phase + static electric interaction for HyP and hydrophobic interaction for Choles)
Column Characterisation
With the plethora of stationary phases available, it can be daunting to know which stationary phases to evaluate. Within SFC, there isn’t a “gold standard” which is typically used, such as in LC methods employing C18 columns.
Lesellier et al have used linear solvation energy relationships (LSER) to observe the analyte retention to properties of the stationary phase under SFC conditions to provide some understanding of the retention mechanisms of the column2. The LSER model is based on 5 Abraham descriptors (E, S, A, B and V) with additional terms included to account for charged analytes (D+ and D-).
These terms quantitatively describe solute / solvent interactions and will be specific to each stationary phase. The information can be presented in spider diagrams such as that in Figure 4, where each vector corresponds to each of the descriptors above. The Shimadzu range of 14 SFC columns were evaluated against a range of other SFC compatible columns.
A dot, which represents a column, in close proximity to another dot indicates similar retention mechanisms (i.e. Diol is close to the HyP), whereas a dot which is far away would suggest different retention mechanisms and potentially vast selectivity differences (i.e. GIS II vs the Amide). A marker which is close to a specific vector also intimates that parameter is a dominant mechanism for that column (i.e. NH2 column along the “A” vector, which refers to hydrogen bonding acidity – the solute’s ability to donate a hydrogen bond).
These sorts of spider diagrams are useful for selectivity different stationary phases for method development, or for identifying potential stationary phases with similar selectivity for back up columns.
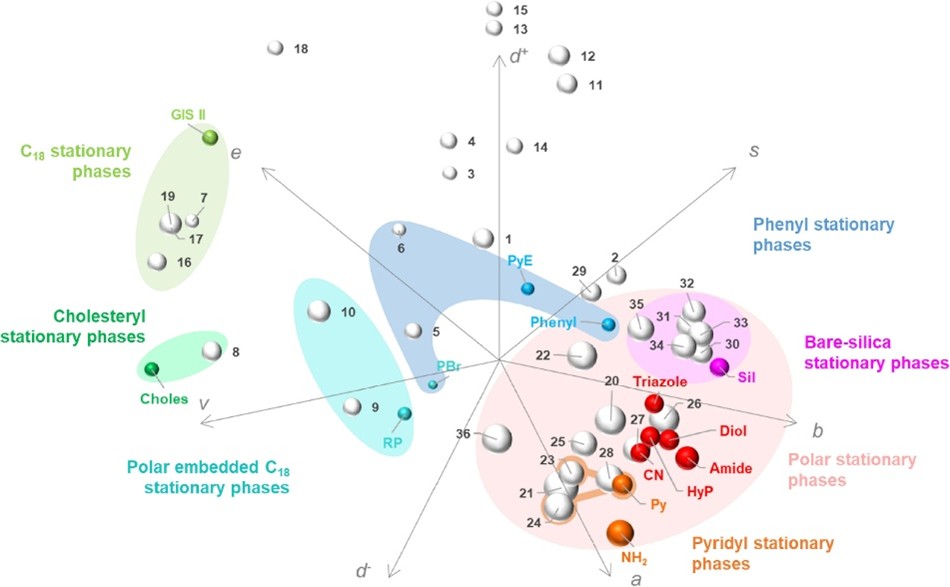
Fig. 4 Spider diagram based on the 7 interaction terms in the LSER model for 14 Shimadzu UC columns and 36 other stationary phases. See Ref [2] for more information

SFC Column Considerations
(1) Due to the pressure on both the inlet and outlet ends, selecting the best hardware for SFC preparative columns is particularly challenging. A frit or bed failure in a preparative SFC column can send packing material into the instrument. Also, rapid CO2 decompression can easily damage preparative columns. Look for hardware and techniques that ensure packed-bed stability for the columns.
(2) Using columns for both HPLC and SFC requires special care regarding purging column liquids after the completion of analyses. After using the column for SFC analysis, reduce the pressure inside the column to vaporise any CO2 in the column or purge it with methanol or ethanol before disconnecting the column.
(3) Disconnecting the column with only supercritical CO2 dries out the packing material due to evaporation of liquid in the column. If that column is then used for HPLC, the high viscosity of the solvent used for the LC mobile phase, such as methanol or water, will cause surface tension around pores in the stationary phase that prevents penetration into the pores and interaction with functional groups.
(4) The supercritical fluid used for SFC analysis has lower viscosity than mobile phase solvents used for HPLC, thus it can penetrate inside pores and interact with functional groups without being affected by surface tension, even if not filled with solvent.
(5) If a column used for HPLC analysis is used for SFC, any water in the column must be thoroughly purged with methanol or ethanol, because the water will not mix with the supercritical CO2.
(6) For SFC, the same mobile phase parameters can be used for everything from normal phase columns to reversed phase columns. First fix the mobile phase parameters, then check separation using multiple columns.
(7) The multiple columns, solvent delivery parameters and separation parameters (such as temperature and pressure) result in a large number of possible parameter combinations. To reduce the amount of work involved in considering separation parameters, use a method scouting system to automatically set and execute respective parameter combinations.
Sample Considerations
Care should be taken with large injection volumes, particularly for preparative SFC. In these situations, the injected sample can plug the column and shut down the chromatographic system. There are several ways of avoiding this “crashing out,” such as using a mobile phase co-solvent like acetonitrile or methanol or mixing the injected sample with solvent that is more compatible with CO2
Rule of thumb – if it can dissolve in methanol, or less polar solvents, it is compatible with SFC.
That is all for this section of the course. Throughout this session, we have learned about the effect stationary phase can play in changing retention mechanisms, and its importance to evaluate multiple stationary phases to determine if there is a suitable phase chemistry. There isn’t a gold standard as in reversed phase chromatography with the C18 ligand functionality. Next week we will look at the effect of the mobile phase in SFC, paying particular attention to the organic modifiers and the additives. See you all soon.
Your Shimadzu Chromatographic Team
References
1 Comparison of Retention Behaviour between Supercritical Fluid Chromatography and Normal-Phase High-Performance Liquid Chromatography with Various Stationary Phases Molecules 2019 Jul; 24(13): 2425
2 Characterisation of stationary phases in supercritical fluid chromatography including exploration of shape selectivity, Q. Gros, J. Molineau, A. Noireau, J. Duval, T. Bamba, E. Lesellier, C. West, J. Chromatogr. A., 2021, 1639, 461923
Mini Quiz
(1) Complete the sentence “if it can dissolve in ____, or less polar solvents, it is compatible with SFC”
Water
Methanol
Glycerol
Chloroform
(2) Why should a column be flushed in 100% methanol if used on both a HPLC and SFC instrument?
Column is most stable stored in methanol
To flush the water out of the column before introducing CO2 as they are not miscible
To improve separation efficiency
To prevent contamination
(3) What are the three terms in the fundamental resolution equation? (Select three)
Efficiency
Stability
Retention factor
Selectivity
Sensitivity
Scroll down for the answers!
Related Resources
-
-
Learn how to troubleshoot common LC issues...
Answers!
1) Complete the sentence “if it can dissolve in ____, or less polar solvents, it is compatible with SFC”
Methanol ü
(2) Why should a column be flushed in 100% methanol if used on both a HPLC and SFC instrument?
To flush the water out of the column before introducing CO2 as they are not miscible ü
(3) What are the three terms in the fundamental resolution equation? (Tick three)
Efficiency ü
Retention factor ü
Selectivity ü