Basics of Total Organic Carbon

What is "TOC"?
Total organic carbon (TOC) indicates the total amount of carbon from organic material present in a sample. The advantages of TOC analysis are the fast analysis time of a few minutes, the exact and matrix-independent quantification and the very low consumption of chemicals. Since this is a sum parameter, the method is not suitable for identifying individual organic components. TOC is mostly determined in liquids where it serves as a representative index for water quality but can also be measured in solids.
Due to the sheer number of known organic compounds, biochemical oxygen demand (BOD), chemical oxygen demand (COD) and permanganate consumption tests have been used in the past as indices for the collective measurement of all organic substances, regardless of their nature.
How is TOC measured?
Carbon Species and Determination Methods
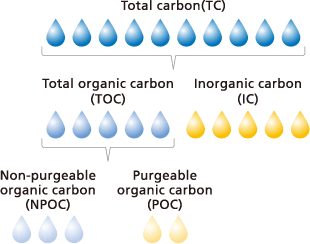
The total amount of all carbon present in a sample is referred to as “total carbon” (TC). It can be further distinguished into two major groups, total organic carbon (TOC) and inorganic carbon (IC). Total organic carbon can be further classified as either non-purgeable organic carbon (NPOC) or purgeable organic carbon (POC).
With regard to the solubility of organic substances in water, a distinction can be made between dissolved organic carbon (DOC), which are substances that pass through a filter with a pore size of 0.45 µm, and particulate organic carbon.
Two main TOC determination methods are used:
Difference method: TOC is determined by subtracting results for TC and IC (TOC = TC - IC).
Direct method: TOC is determined by measuring NPOC, in other words TC after IC removal (TOC = NPOC).
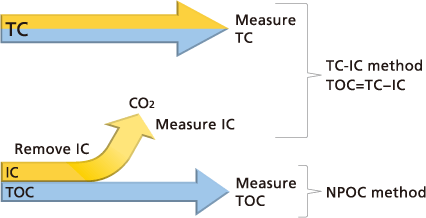
Measuring IC
For TOC measurement, IC refers to the total sum of inorganic carbon contained (where CO₂ indicates dissolved carbon dioxide, HCO₃‾ bicarbonate ions, and CO₃²‾carbonate ions). The quantity of dissolved carbon dioxide, bicarbonate ions, and carbonate ions in water are kept in an equilibrium that depends on the pH level of the water, according to the expression below.
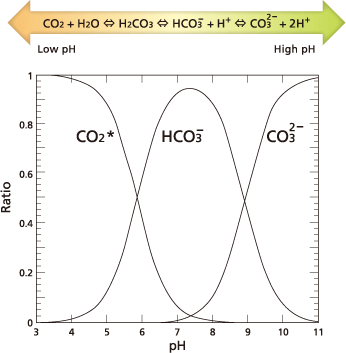
With decreasing pH, the equilibrium moves to the left side of the diagram above. At a pH of 3 or lower, almost all IC becomes dissolved carbon dioxide, which is easy to strip from water.
Based on that principle, IC is measured by acidifying the sample to pH < 3 and then measuring the CO₂ extracted from the sample by stripping with CO₂-free air.
Using Direct and Difference Methods
Both the Difference Method (TC - IC) and the Direct Method (TOC = NPOC) are used to measure TOC. However, the optimal method must be selected based on sample characteristics.
The difference method requires two separate analyses and is therefore subject to a larger measurement error than the direct method due to error propagation. As a guideline, the TOC content of the sample must also be greater than the IC content, otherwise the measurement uncertainty becomes unacceptable for the purpose of the analysis.
For samples that are prone to foaming, or that have significant volatile contents, for example, the TC - IC method is used because the NPOC method can result in loss of purgeable organic carbon (POC) from samples during the stripping step, or in general due to foaming ingredients.
TOC Oxidation methods
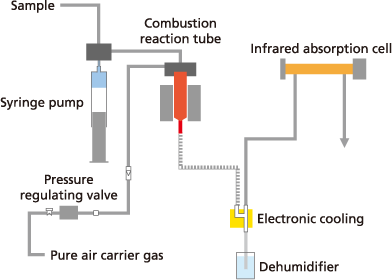
TOC analyzers are, in general terms, CO₂ gas analyzers with an upstream oxidation stage and sample preparation system. Regardless of which TOC determination method is used, TOC (also TC) is measured by the oxidation of organic carbon and subsequent quantification of the resulting CO₂ using an infrared detector. There are different oxidation methods for conversion to CO₂, of which two have become established; combustion oxidation and wet oxidation.
Combustion oxidation method
The sample is injected into a high-temperature (650 to 1,200 °C) combustion furnace to incinerate all organic carbon in the sample and measure it as fully oxidized carbon dioxide. Due to the simplicity of using heat/combustion as the principle for oxidation, the method requires no reagents for pretreatment or posttreatment processes. One of the main features of this method is its ability to efficiently oxidize organic carbon matter that is otherwise resistant to decomposition, such as particulate matter or macromolecular organic substances. In the past, high temperatures (1000 °C and more) were required because the first TOC instruments used peak height for integration. There, the conversion to CO₂ had to be extremely fast so that the signal was recorded as sharp as possible to achieve the best possible resolution.
Very high combustion temperatures lead to the formation of salt melts in the analyzer, which in turn cause increased maintenance due to deactivation of the catalyst, corrosion of the combustion tube and the detector cell. Salt interference on the detector cell from the molten salt products can affect the quality and accuracy of the data. In addition, maintenance time is extended due to the longer cooling and reheating time required because of the higher combustion temperature.
Shimadzu has developed the high-temperature catalytic oxidation (HTCO) at 680 °C. While the platinum catalyst ensures complete conversion of all carbon components, the combustion temperature is below the melting points of common salts. Thus, problems caused by salt are minimized while excellent recovery rates are achieved for all organic components. TOC combustion oxidation can easily be expanded to include the determination of an additional sum parameter for nitrogen, Total bound Nitrogen (TNb).
Wet Oxidation method
With this method, an oxidizing agent is added to samples to chemically decompose carbon in organic matter for measurement as carbon dioxide. Though heat (up to 100 °C) or ultraviolet irradiation can be applied to promote the oxidation reaction, the ability of the chemical reaction to oxidatively decompose matter is weaker than combustive oxidation, which tends to result in lower carbon recovery rates from suspended or other particulate organic matter, or persistent substances. It does however allow the injection of comparably larger amounts of sample to reach lower limits of detection.
Due to its superior oxidative reaction, the combustion oxidation method is commonly used to measure TOC levels in environmental water, factory effluent, and similar samples, where water samples often contain large amounts of insoluble organic carbon.
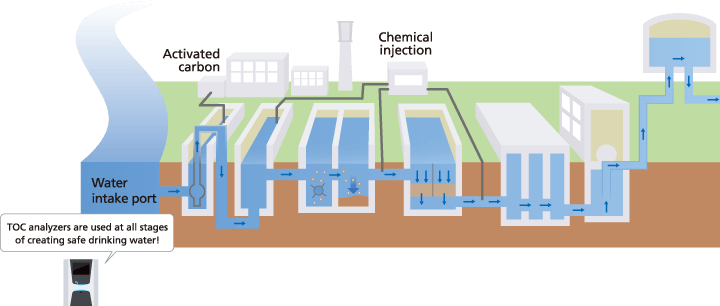
TOC in Drinking Water
Confirming the safety of public drinking water
Public drinking water is supplied by using a water treatment based on the water quality of the given river, lake, groundwater, or other water source. However, the water quality of public drinking water can vary due to changes in the water quality or usage rate of the river or lake.
Therefore, it is important to regularly inspect the safety of treated water.
Reactions between organic matter and disinfectants used for water treatment are said to generate substances that are harmful to humans. Therefore, measuring TOC in public drinking water provides an important index for confirming the safety of public drinking water.
The TOC level is also said to affect the taste of public drinking water, so it can be used as an index for how good public drinking water tastes.
Water Treatment Management
A variety of processes are used to eliminate microorganisms and organic matter from water at water treatment plants.
Measuring the TOC level at each process step can be used to confirm that each process is functioning properly. (pH and turbidity values are also measured in addition to TOC.) In addition to confirming water treatment functions, measuring TOC can also help optimize water treatment. Adjusting the amount of chemicals used based on the TOC values measured at each process step can also help reduce water treatment costs.