Basics of Supercritical Fluid Chromatography
SFC Basics Course
1 - What are Supercritical Fluids and the History of SFC
2 - SFC Instrumentation and Advantages of SFC
3 - SFC Analytical Operating Conditions Part I Stationary Phases
4 - SFC Analytical Operating Conditions Part 2 Mobile Phases and Other Parameters
5 - Preparative SFC
Welcome Back!
Welcome back to this next instalment of the Basic Introduction to SFC course! In the last session, we looked at what a supercritical fluid was and a brief history of the technique. In this instalment, we will be looking at the modules which make up an SFC instrument and the analytical advantages of employing SFC.
SFC Components
SFC systems involve roughly the same configuration of instruments as HPLC systems. Fig. 1 shows a comparison of flow diagrams for SFC and HPLC systems. The four major differences between SFC and HPLC configurations are described below.
- Pumps Designed Specifically for Pumping Supercritical Carbon Dioxide
The carbon dioxide used for SFC is liquified by cooling. Therefore, the delivery pump must have built-in cooling functionality.
-
- Back Pressure Regulator (BPR)
A unit is required to keep the CO2 in a solvent state (liquid or supercritical fluid) within the system and prevent it from vaporising by maintaining the pressure level within flow channels. In an SFC system, the BPR unit is positioned downstream from a UV, PDA, or other detector or upstream from an evaporative light scattering detector (ELSD) or mass spectrometer (MS).1 The BPR unit detects pressure in the flow channels and then rapidly opens and closes valves to maintain a constant pressure within the flow channels.
-
- Modifier Delivery Pump
For SFC analysis, an organic solvent such as methanol or acetonitrile (modifier) is pumped for mixing with the supercritical carbon dioxide. That means separate pumps are required for pumping the supercritical carbon dioxide and modifier.
-
- Make-up Delivery Pump
If an ELSD or MS is used for detection or if the system is used for preparative separation, then a solvent (make-up solvent) is pumped to prevent precipitation in the flow channels or to improve the recovery rate of components in separated fractions. Make-up solvent is also pumped to improve sensitivity during MS detection, because supercritical carbon dioxide does not promote ionisation
-
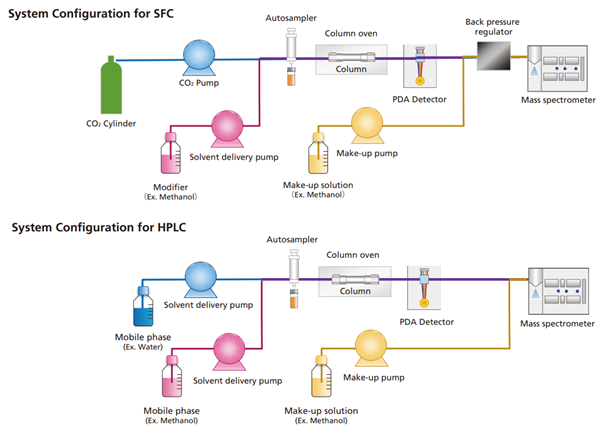
Fig. 1 Comparison of HPLC and SFC system configurations
Shimadzu’s SFC system product line includes a variety of systems, such as SFC-UV systems that use UV (or PDA) detection, SFC-MS (Fig. 2) systems that include a mass spectrometer capable of SFC speeds, and screening systems that can automatically switch between multiple columns and modifiers for considering various analytical conditions.
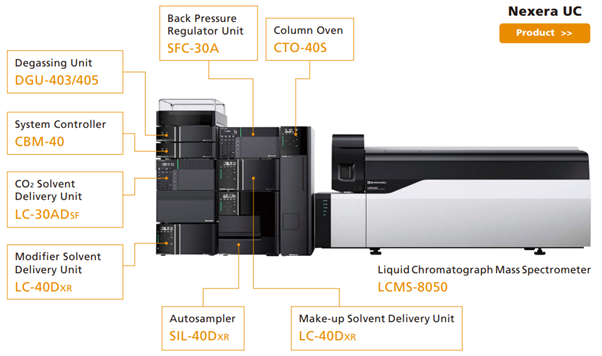
Fig. 2 Nexera UC SFC-MS system
If both UHPLC and SFC are available during analytical condition development, optimal separation parameters can be selected from a wider range of potential separation parameters. There are various instruments which are designed to seamlessly switch between LC and SFC, allowing both types of analyses to be performed on a single system Fig. 3 shows the flow diagram of the Nexera UC/s switching system, which integrates both UHPLC and SFC capability in a single platform Both UHPLC and SFC analyses can be performed by switching between solvent delivery units and switching ON or OFF the pressure control mode of the back pressure control valve. That can reduce installation space and initial cost and increase the instrument utilisation rate. By using the mobile phase solvent switching valve in combination with the column switching valve, mobile phase conditions can be changed automatically and continuously for up to twelve columns, which enables comprehensive measurements that improve method development efficiency.
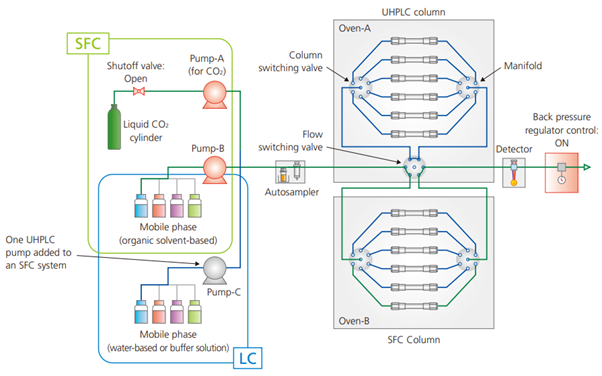
Fig. 3 Flow diagram for Shimadzu's SFC/UHPLC switching system, the Nexera UC/s (Green line indicates sections used during SFC)
Analytical Advantages of SFC
The supercritical carbon dioxide used as a mobile phase for SFC offers unique advantages. Five of these advantages are described below.
- (1) Low polarity
- The polarity of supercritical carbon dioxide is similar to n-hexane. Normal phase chromatography is a separation method that uses a solvent with low polarity, such as n-hexane, as the mobile phase, and a substance with high polarity, such as silica, as the stationary phase. One advantage of normal phase chromatography is it offers better structure recognition ability than reversed phase chromatography. SFC uses supercritical carbon dioxide, which has similar properties as n-hexane, and also offers excellent structural recognition similar to normal phase chromatography (Fig. 4). By using a column designed for chiral chromatography, which is packed with a stationary phase with optically active functional groups, SFC is suitable for separation of chiral compounds.
-
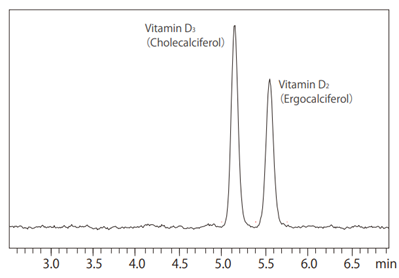
Fig. 4 Separating vitamins D2 and D3 (UC-Pye column)
- (2) Miscibility with Other Organic Solvents
- Supercritical carbon dioxide can be mixed with certain organic solvents, such as methanol, ethanol, isopropanol (IPA), acetonitrile, and tetrahydrofuran (THF). In contrast, n-hexane, which offers low polarity similar to supercritical carbon dioxide, cannot be mixed with methanol and similar solvents, due to its relatively high polarity. By mixing the supercritical carbon dioxide with such modifiers, SFC can achieve a wide variety of separation patterns. This allows comprehensive analysis for a wide range of low-to-high polarity compounds not previously possible with HPLC. For example, earlier GC was required for analysing fatty acids and HPLC for glycerides. With SFC, both fatty acids and glycerides can now be analysed simultaneously due to the hexane-like properties of the supercritical carbon dioxide in combination with a column designed for reversed phase chromatography (Fig. 5).
-
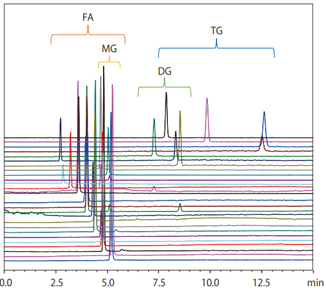
Fig. 5 Example of simultaneously analysing fatty acids and triglycerides by SFC
- (3) Low Viscosity
- Supercritical carbon dioxide has lower viscosity than solvents such as water, and a high diffusion coefficient. That results in lower column back pressure, assuming the column has the same internal diameter and length, is packed with the same size particles, and supercritical carbon dioxide is pumped through the column at the same rate. That enables analysis with a high linear velocity, which can speed up analysis times. Fig. 6 shows a comparison of linear velocity and column pressure plots for HPLC and SFC.
-
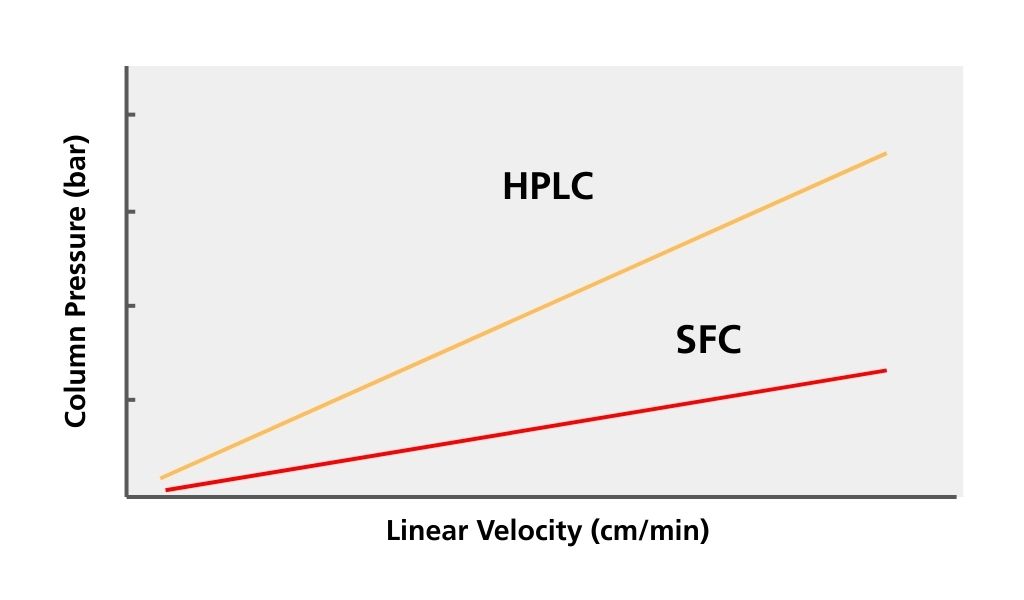
Fig. 6 Linear velocity vs column back pressure
Though higher linear velocity can shorten HPLC analysis time, it results in increased column pressure. The upper range of column pressure is limited by instrument design and column capability. CO2 in the supercritical state has lower viscosity than water and other organic solvents typically used for HPLC. That means shorter analysis times are possible for SFC than HPLC without loss of resolution and change in selectivity.
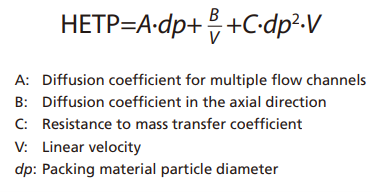
Supercritical carbon dioxide also has a higher coefficient of diffusion than solvents used for LC. The diffusion coefficient contributes to determining how quickly compounds reach equilibrium between stationary and mobile phases during chromatography and affects resolution. The van Deemter equation below indicates the relationship between physical parameters that determine the height equivalent to a theoretical plate (HETP) value, which is used to indicate column resolution.
-
-
Plots of the van Deemter equation for HPLC and SFC are shown in Fig. 7.2 In the formula above, Term A is based on the multi-flow diffusion generated by the packing material in the column and is proportional to the packing material particle size.
Term B is determined by the molecular diffusion in the axial direction of the column. This diffusion phenomenon occurs while compounds stagnate in the stationary or mobile phases and is inversely proportional to linear velocity, regardless of particle size.
Term C is derived from how easily compounds can penetrate pores in the packing material and the mass transfer diffusion that contributes to linear velocity. The mass transfer diffusion value is proportional to linear velocity and the square of the particle diameter, which results in higher HETP values for small particles with low linear velocities. In the case of HPLC, mobile phases with a high linear velocity will result in larger mass transfer diffusion values, but supercritical carbon dioxide has a higher diffusion coefficient than HPLC solvents and can penetrate packing material pores more easily. That results in lower mass transfer diffusion values even for high linear velocities.
-
That makes SFC an analytical technique that can achieve ultra-fast analysis without sacrificing resolution even at high linear velocities.
-
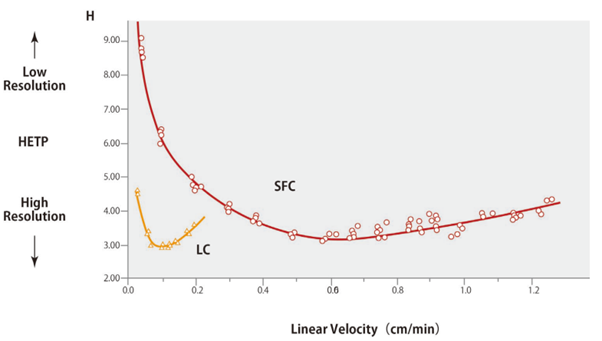
Fig. 7 van Deemter plot comparison for LC and SFC
These plots indicate the regions where the optimal linear velocity for SFC is higher than for HPLC. An example comparing the analysis of tocopherols by HPLC and SFC is shown in Fig. 8.3
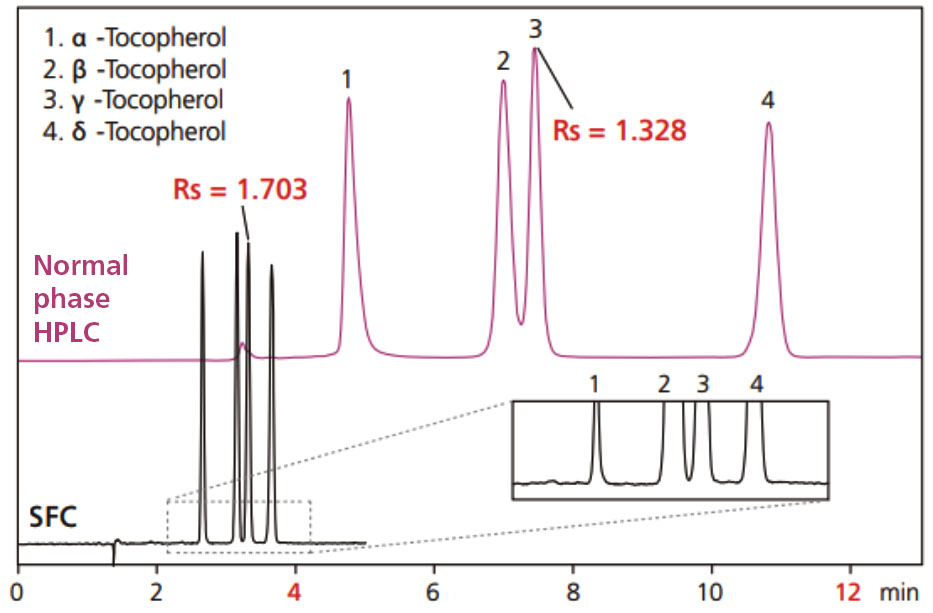
Fig. 8 Comparison example of analysing tocopherols by HPLC and SFC
- (4) Vaporisation
- Supercritical carbon dioxide evaporates at a temperature and pressure lower than the critical point. Under certain temperature and pressure conditions, CO2 will become a solvent, which is used as a mobile phase for SFC. If an MS is used as a detector, then a back pressure regulator is installed just before it so that only the modifier or make-up solvent reaches the MS. Consequently, unlike conventional LC-MS analysis, this reduces the diluting effects of the mobile phase, potentially increasing the sensitivity while using SFC-MS. This can be seen in the example below where 400 components were analysed with 90% of these showing higher sensitivity with SFC-MS (Fig. 9). Fig. 10 shows a comparison of results from using LC-MS and SFC-MS to measure samples prepared to 10 ppb.
-
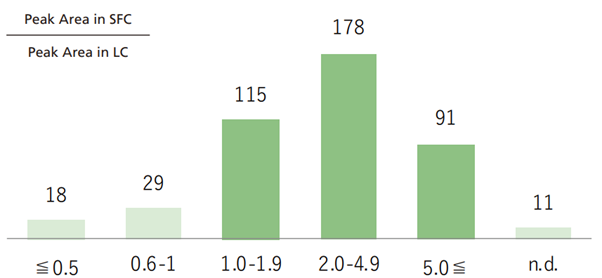
Fig. 9 Area comparison for LC-MS and SFC-MS
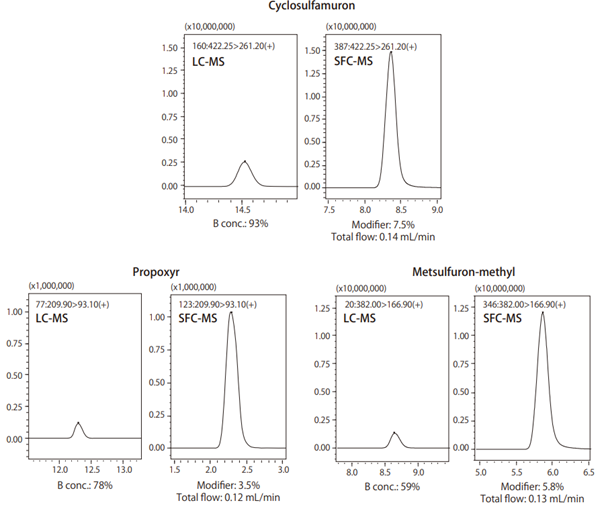
Fig. 10 Comparison of mass chromatograms of standard pesticide mixture solution measured by LC-MS and SFC-MS
-
- (5) Solvent Cost
- The similar polarity of supercritical CO2 and n-hexane makes the former appropriate to be used for normal phase separations. Normal-phase chromatography consumes large amounts of solvent. However, because SFC consumes less solvent and involves treating less liquid waste, the overall solvent cost is lower than for HPLC (Fig. 11)
-
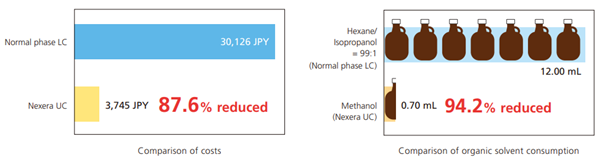
Fig. 11 Comparison of analytical costs and solvent consumption quantities for HPLC and SFC
- (6) Wide Polarity Range for Samples
- During the infancy of SFC, applications were typically aimed at apolar, fatty compounds. However, the addition of organic modifiers such as methanol to the mobile phase has expanded the polarity range available for SFC, thereby covering the normal phase and reversed phase range of compounds. Gradients are typically run from 5-40% modifier. This has been further expanded in recent years to include more polar compounds in the typical HILIC range. This expansion is possible with the addition of water (1-10%) in the organic modifier, and the gradient range extended to 100% organic modifier. This would eliminate the need for several methods to analyse a broad range of analytes in a sample.
-
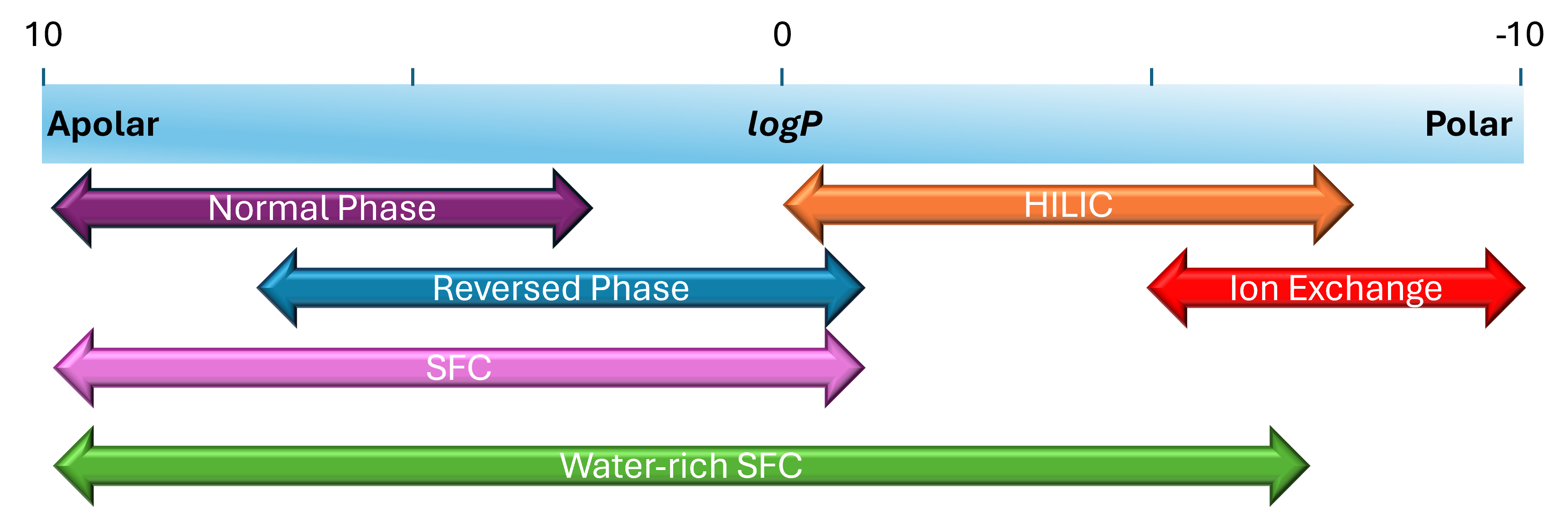
Fig. 12 Polarity range covered by different LC techniques compared to UHPSFC and water rich UHPSFC/UC. Image adapted from Reference 4
-
- (7) Lower Backpressure
- The lower viscosity of the mobile phase produces a lower backpressure. This allows for small particle sizes not conventionally employed in HPLC but can be used for SFC methods. For UHPLC applications, 1.8 μm particle morphologies can be used but high backpressures can be expected (>850 bar). These same size particles would have a substantially lower backpressure under SFC conditions. It is also feasible that smaller particle sizes such as 1.3 μm could be used to maximise efficiency in SFC.
In the next two sessions of the course, we will investigate common operating parameters used in SFC analytical methods, specifically column functionalities next week.
Your Shimadzu Chromatographic Team
References
1Overview of the retention in subcritical fluid chromatography with varied polarity stationary phases, E. Lesellier, J. Sep. Sci. 2008, 31, 1238-1251
2The dependence of reduced plate height on reduced velocity in carbon dioxide supercritical fluid chromatography with packed columns P.A. Mourier, M.H. Caude, R.H. Rosset, Chromatographia, 1987, 23, 21-25
3Shimadzu Technical Report “Supercritical Fluid Chromatography” (C190-E200)
4Metamorphosis of supercritical fluid chromatography: A viable tool for the analysis of polar compounds? G.L.Losacco, J. Veuthey, D. Guillarme, TrAC Trends in Anal. Chem., 2021, 141, 116304
Mini Quiz
(1) What is the role of the back pressure regulator?
To increase the flow rate of the system
To keep the carbon dioxide in the supercritical state
Removes impurities
To remove pressure from the system
(2) Does CO2 have a lower viscosity than water in the supercritical state?
Yes
No
(3) Is pure CO2 miscible with water?
Yes
No
Scroll down for the answers!
Related Resources
-
-
Download your guide!
Answers!
(1) What is the role of the back pressure regulator?
To keep the carbon dioxide in the supercritical state ü
(2) Does CO2 have a lower viscosity than water in the supercritical state?
Yes ü
(3) Is pure CO2 miscible with water?
No ü




