Basics of Supercritical Fluid Chromatography
SFC Basics Course
Welcome Back!
Welcome back to this SFC basics introduction course. We hope you have found it informative and useful to begin your journey into SFC. In the previous sessions, we have looked at the analytical advantages of SFC, the modules required to run an SFC, as well as the history of SFC and what makes a supercritical fluid. In this final part of the course, we will look at using SFC in a preparative system.
Preparative SFC
GC and HPLC are commonly used to pre-separate samples, but SFC can be used in the same way as well. Preparative separation by SFC offers the following advantages.
- (1) Posttreatment after Preparative Separation
Supercritical carbon dioxide evaporates at ambient temperature and pressure conditions, which eliminates the need for posttreatment.
- (2) Solvent Cost for Large Preparative Separation Quantities
Using inexpensive and environmentally-friendly CO2 can reduce the cost of purchasing and disposing of solvents.
- (3) Preparative Separation Recovery Rates
After preparative separation, HPLC requires posttreatment steps, such as solvent evaporation or concentration. In contrast, SFC solvents evaporate easily, which minimises the need for posttreatment and prevents component fragmentation or decomposition during posttreatment.
-
- When SFC is used for preparative separation, an organic solvent capable of dissolving the target components is sometimes added after column separation to prevent their precipitation within flow channels. Gas-liquid separators can then be employed to remove these solvents, and a variety of such devices are currently being developed by various manufacturers.
-
Similar to preparative HPLC, preparative SFC begins with analysis-scale optimisation before scaling up. This involves switching to a preparative column, adjusting the mobile phase flow rate, and modifying the sample injection volume. Equivalent separation can be achieved by increasing flow rate and injection volume in proportion to the column’s cross-sectional area. Fig. 1 shows an example of changing from an analysis scale to a preparative scale. By using a column with the same stationary phase, separation equivalent to the analysis scale can be achieved.
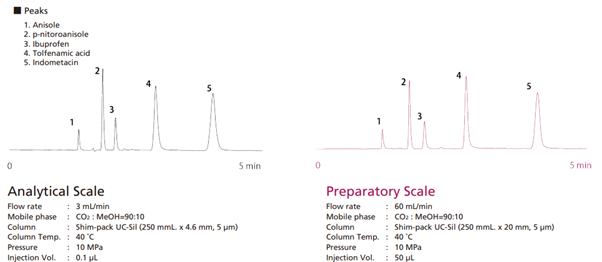
Fig. 1 Migrating from analysis scale to preparative scale with SFC
Preparative LC is a technique used to purify samples for specific target components. It is used in a wide range of fields, including chemicals, pharmaceuticals, and food testing. Preparative LC serves as a powerful tool for achieving higher purity and recovery rate levels of target components, but it requires drying and powderising steps.
SFC can improve preparative workflow efficiency by significantly reducing the amount of work involved in the powderisation process after preparative purification. The Nexera UC product line includes three systems—a stacked fraction system intended for large-volume fractionation, a multi-fraction system for separating multiple peaks, and an analytical fraction system intended for analysis-scale fractionation.
These products also feature Shimadzu’s unique gas-liquid separator (patented) that inhibits sample dispersion and carryover to obtain high recovery rates. Therefore, excellent recovery rates can be achieved even for highly volatile compounds, such as the fragrance linalool, regardless of the flow rate or modifier concentration. The following sections describe the three product lines and the gas-liquid separator.
- Stacked Fraction System
This system is optimised for large volume fractionation that involves repeatedly injecting compounds with up to several components (Fig. 2). The FRS-40 injection and collection unit can function as both an injector and fraction collector for repeatedly injecting the same sample to collect gram-level preparative fractions. It can inject volumes up to 20 mL* and collect ten fractions. It supports flow rates from 10 to 150 mL/min and connecting columns with an internal diameter from 10 to 30 mm.
-

Fig. 2 Nexera UC Prep stacked fraction system
- Multi-Fraction System
This system is suitable for applications involving fractionation of multiple components in samples with many peaks detected, such as for impurities in pharmaceuticals (Fig. 3). Volumes up to 2 mL* can be injected using an autosampler that holds up to 162 samples (when using 1.5 mL vials). The FRC-40 SF fraction collector can collect up to 540 fractions (using 10 mL vials). It supports flow rates from 10 to 150 mL/min and connecting columns with an internal diameter from 10 to 30 mm.
-

Fig. 3 Nexera UC Prep multi-fraction system
- Analytical Fraction System
This system enables analysis-scale fractionation for applications that only require fraction quantities up to several milligrams, such as for checking synthesis (Fig. 4). By connecting an FRC-40 SF fraction collector to the Nexera UC system, the same system can be used for applications ranging from determining analytical conditions by method scouting to preparative separation of about a few milligrams. With a maximum flow rate of 5 mL/min, it supports using analytical size columns.
-

Fig. 4 Nexera UC Analytical fraction system
- LotusStreamTM Gas-Liquid Separator
When carbon dioxide transitions from the supercritical fluid state to the gas state during preparative SFC, its volume immediately expands by about 500 times, which can cause eluate to splatter from the column, a factor leading to lower recovery rates. The newly developed LotusStreamTM (patented) gas-liquid separator (GLS) uses multiple flow channels to limit the flow rate without increasing the tubing diameter. As a result, the carbon dioxide is discharged externally and the liquid travels along the column and then drips directly below without the eluate splattering (Fig. 5). This improves the recovery rate without compromising on sample dispersion and carryover. There is also a LotusStreamTM GLS available for the analytical scale, which allows for fractionation of small volumes, even into vessels such as 1.5 mL vials. The volatile optical isomers of linalool in Fig. 6 illustrates high purity fractionation using the LotusStreamTM separator.
-

Fig. 5 Gas-liquid separation with and without the LotusStreamTM Separator
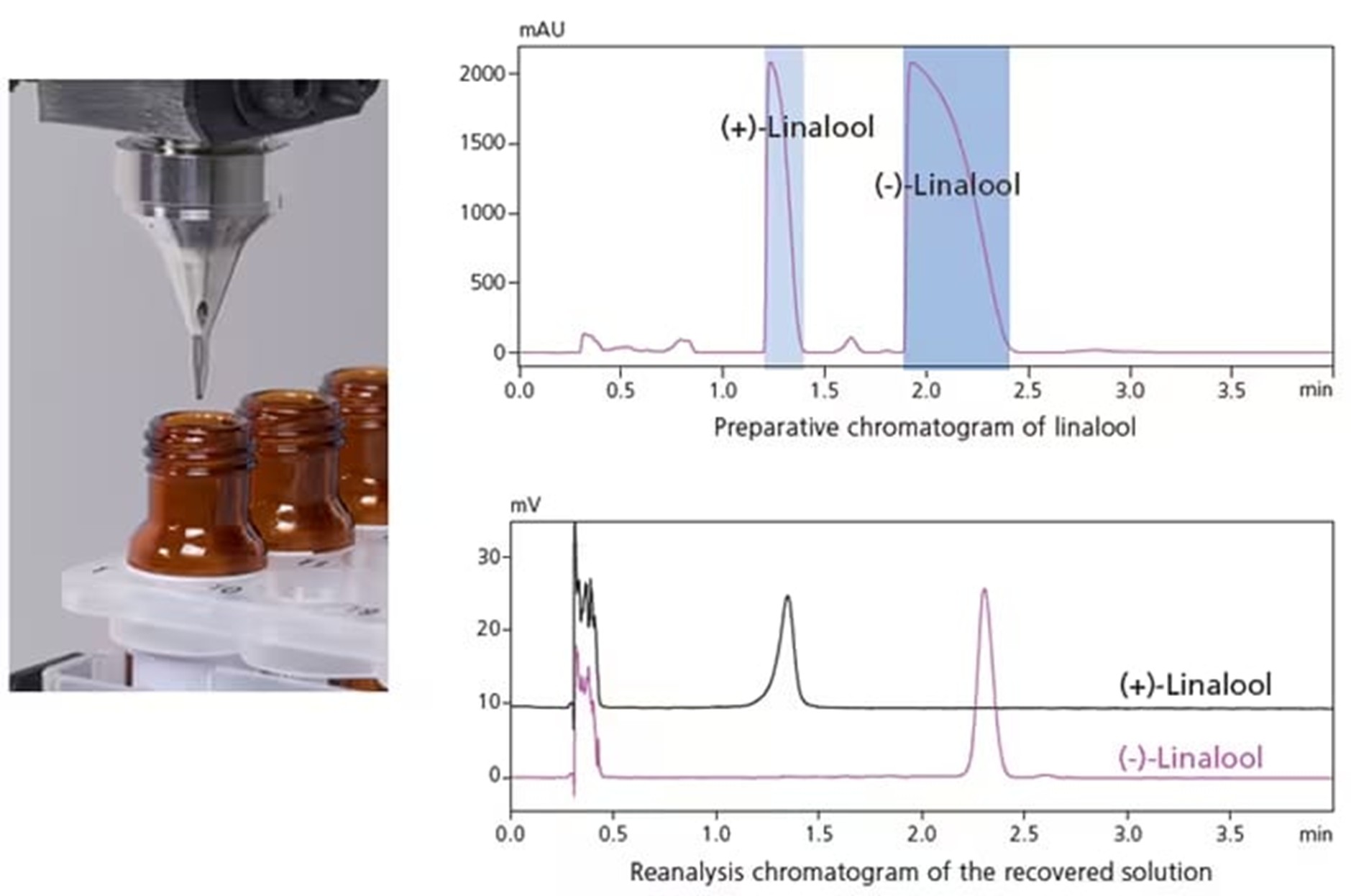
Fig. 6 Fractions of the optical isomers of linalool reanalysed after using the LotusStreamTM

Prep Workflow
The workflow for preparative analysis is similar between both HPLC and SFC modalities (Fig. 7). The sample is first chromatographed using a scouting gradient. This is then optimised to meet the separation criteria, which means adjusting the %B/min change, the temperature, the backpressure regulator settings and other operating parameters. Upon completion of the method on the analytical scale, a loading study should be performed to ensure there isn’t any issue with the chromatography on a larger scale based on sample concentration. Finally, the method can then be scaled up geometrically for the new column dimensions by adjusting the injection volume and flow rate so the compound of interest can be purified.
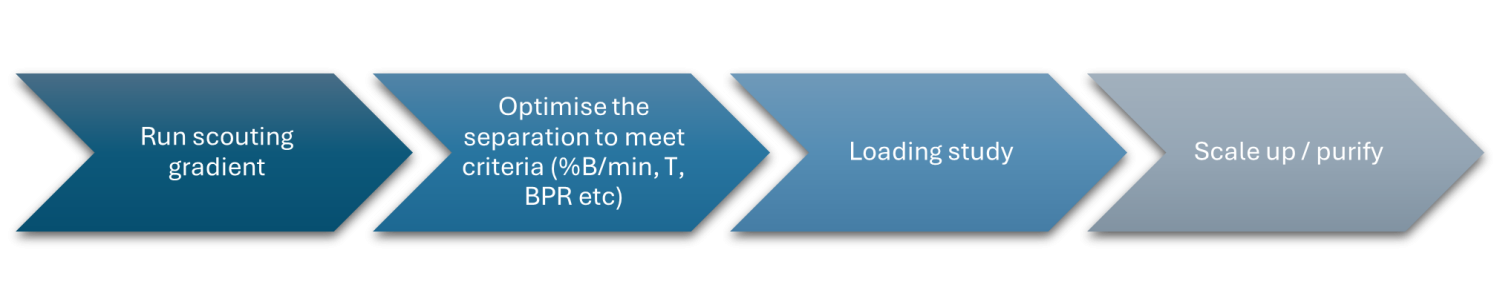
Fig. 7 Simple flow diagram for the preparative process
Prep Considerations
- (1) Volume of CO2.
The amount of supercritical CO2 needs to be carefully controlled. It is important to have a CO2 monitor installed to detect gas leaks. This is relevant for both prep and analytical scale.
- (2) Stationary phase chemistry.
Ensure that the analytical scale stationary phase is available in the preparative scale format.
- (3) Loading studies.
It is important to perform loading studies from an analytical scale up to prep scale. It is important to know about the solubility of the sample in question. The injection volume is increased incrementally until acceptable resolution has been lost or loss of pure fractions.
- (4) Scaling.
The injection volume and flow rate are geometrically scaled to maintain peak shape.
Frequently Asked Questions
Are there any special precautions for indoor use? Are there any special precautions for indoor use? Are there any special precautions for indoor use?
Be careful to discharge exhaust gases into a fume hood to prevent CO2 concentrations from becoming too high within the laboratory. Installing a CO2 meter is also recommended for the purpose of detecting CO2 gas leaks within the room.
Is a temperature-controlled (and humidity-controlled) room environment preferable for maintaining the carbon dioxide gas supply temperature?
As long as the instruments are used within the manufacturer’s recommended conditions, controlling the room temperature is probably not a significant concern because temperature is controlled in the column oven, but gas cylinders are affected by the ambient temperature where they are installed so care should be given to that temperature.
Can an LC unit currently in use (for example? Prominence™ LC-20A series) be used in combination with SFC?
Yes, if the current Prominence unit is operated in combination with a modifier pump. For more details, contact Shimadzu.
Can CO2 cylinders that are typically distributed be used?
Cylinders of 99.9 % CO2 gas that are typically sold are more than acceptable for use. However, quality can vary so be sure to contact the gas vendor to confirm detailed specifications. A higher quality is required for MS applications.
About how much CO2 gas is used per analysis?
Assuming ten minutes per analysis and pumping 100 % CO2 at a flow rate of 3 mL/min, about 30 g is used per analysis. Use that value to recalculate usage based on appropriate changes to the analysis time, modifier ratio (which reduces the proportion of CO2), and flow rate
Do samples analysed by SFC need to be dissolved in a liquid like HPLC?
In general, samples must be dissolved in a solution before injection, just like for HPLC. However, by connecting it to a supercritical fluid extraction (SFE) system, even components in solid samples can be analysed.
For SFC, how is sample solubility in the mobile phase checked?
Due to the polarity of supercritical carbon dioxide, the ideal mobile phase should be n-hexane or a mixture of n-heptane and 2-propanol, but typically samples are dissolved for use in 100 % methanol or another modifier.
Can samples containing water be injected?
Yes, they can. However, for larger injection volumes, the sample solvent could affect peak shape or retention times.
Does supercritical carbon dioxide cause any sample decomposition?
Supercritical carbon dioxide is known to cause slight acidity in mobile phases, but there is minimal risk of decomposing or otherwise altering compounds.
Are there any examples of analysing blood, urine, or other biological samples by injecting them directly into an SFC system?
We are not aware of any examples of analysing blood by direct injection. However, there are examples of analysing blood soaked in filter paper by SFE-SFC and examples of directly injecting urine samples for SFC-MS analysis.2-4
Are there any samples that can be analysed by HPLC but not SFC?
SFC analysis of amino acids, soluble vitamins, polyphenols, and other highly polar compounds is possible by increasing the modifier concentration and adding an acid. However, the resulting peak shapes are not as good as when using HPLC. For preparative applications, SFC offers the advantage of not requiring drying or purification steps after fractionation.
What type of modifier solvent is typically selected for analysing compounds that decompose in alcohol or water?
A non-protic solvent is often used in cases where SFC is useful for analysing samples that decompose or change properties when exposed to moisture. Candidate non-protic solvents include acetonitrile and THF.
What liquid wastes remain after analysis? Do they include the solvent with dissolved analytes, modifiers, and make-up solution?
Yes, waste liquids include the solvent containing dissolved measurement target substances, modifiers, and make-up solutions. However, liquid waste bottles will also contain carbon dioxide gas and some solvent consumption occurs due to evaporation.
What additives can be added to modifiers to improve peak broadening problems when measuring hydrophilic peptides?
There is a report of analysis by using TFA as a method for improving peak shape in peptide analysis by SFC.5.
Can HPLC columns also be used for SFC?
SFC columns are generally compatible with fittings used in Shimadzu HPLC systems so it is possible to connect them. However, confirm the pressure capacity and other requirements for actual use with respective column manufacturers.
Can columns be used for both HPLC and SFC?
Yes, they can. However, it requires particular care regarding purging column liquids after analyses are finished. After using the column for SFC analysis, reduce the pressure inside the column to vaporise any CO2 in the column or purge it with methanol or ethanol before disconnecting the column. Disconnecting the column with only supercritical carbon dioxide dries out the packing material due to evaporation of liquid in the column. Then if that column is used for HPLC, the high viscosity of the solvent used for the LC mobile phase, such as methanol or water, will cause surface tension around pores in the stationary phase that prevents penetration into the pores and interaction with functional groups. Because the supercritical fluid used for SFC analysis has lower viscosity than the mobile phase solvents used for HPLC, they can penetrate inside pores and interact with functional groups without being affected by surface tension, even if not filled with solvent. If a column used for HPLC analysis is used for SFC, any water in the column must be thoroughly purged with methanol or ethanol, because the water will not mix with the supercritical carbon dioxide.
Is there a difference in the service life of columns used for SFC and HPLC?
Due to the lower viscosity of supercritical fluid, it applies less force on the packing material. In other words, it applies less back pressure on the column, which tends to result in longer column life than for HPLC.
Since we cannot predict the elution order for separation by SFC, we do not know which columns are to be used or how they should be used. When screening columns for SFC, what separation parameters should be considered?
For SFC, the same mobile phase parameters can be used for everything from normal phase columns to reversed phase columns. Therefore, we recommend first fixing the mobile phase parameters and then checking separation using multiple columns. Given the multiple columns, solvent delivery parameters, and separation parameters such as temperature and pressure, there is a huge number of possible parameter combinations. To reduce the amount of work involved in considering separation parameters, you are recommended to use a method scouting system because it automatically sets and executes respective parameter combinations.
Are retention time and peak intensity repeatability levels comparable to HPLC levels in terms of precision and accuracy?
Repeatability comparisons for HPLC and SFC using identical conditions have resulted in comparable repeatability and quantitative accuracy levels.
We have been struggling with matrix and ghost peaking problems in our LC-MS analysis. Will the same phenomena occur for SFC as well?
Ionisation can be inhibited by the matrix even for SFC-MS. However, since LC and SFC can result in significantly different separation behaviour, sometimes SFC-MS can resolve such LC-MS matrix effect problems.
Can SFE be used for samples that contain non-volatile salts?
Yes, as long as the non-volatile salts are not dissolved by the extraction solvent. However, beware of contamination and precipitation if using a mass spectrometer for detection
So that is the final session in this introductory SFC course. We thank you again for your participation and wish you much success in the final test. Click on the link below to take you through to the quiz to gain your Certificate of Attendance.
If you have enjoyed this course, then feel free to inform your colleagues, employees, friends, and acquaintances who work with or have an interest in SFC who want to gain a basic understanding. The next start will be announced through our newsletter or on our homepage. We will also be looking at generating a preparative course. To be the first to hear about it, click here.
Your Shimadzu Chromatographic Team
References
1Cross-Pharma collaboration on the development and evaluation of a new mid-scale preparative supercritical fluid chromatography instrument, M. Biba, M. Wong, A. Akin, E.T. Manning, L. Schaffter, L. Miller, Y. Zhang, W. Farrell, J.O. DaSilva, L. Nogle, B. Hritzko, F. Riley, R.P. DePianta, K. Barry, D.A. Gao, E. Seest, M. Goel, L. Chung, J. Paulson, H. Lee, D.B. Moore, S. Dong, W. Leister, N. Fukushima, A. Sasaki, T. Lee, T. Iriki, M. Nishimura, M. Tomita, M. Owa, K. Tanaka, T. Shagawa, T.J. Moran, T. Bamba, C.J. Welch, Org. Proc. Res. Dev., 2020, 24, 1271-1280
2Carotenoids and apocarotenoids determination in intact human blood samples by online supercritical fluid extraction-supercritical fluid chromatography-tandem mass spectrometry, M. Zoccali, D. Giuffrida, F. Salafia, S.V. Giofre, L. Mondello, Anal. Chim. Acta., 2018, 1032, 40-47
3High-throughput phospholipid profiling system based on supercritical fluid extraction–supercritical fluid chromatography/mass spectrometry for dried plasma spot analysis, J. Chrom. A., Uchikata, A. Matsubara, E. Fukusaki, T. Bamba, 2012, 1250, 69-75
4Use of on-line supercritical fluid extraction-supercritical fluid chromatography/ tandem mass spectrometry to analyse disease biomarkers in dried serum spots compared with serum analysis using liquid chromatography/tandem mass spectrometry, M. Suzuki, S. Nishiumi, T. Kobayashi, A. Sakai, Y. Iwata, T. Uchikata, Y. Izumi, T. Azuma, T. Bamba, M. Yoshida, Rapid Comm. in Mass Spec., 2017, 31, 886-894
5Advantageous use of SFC for separation of crude therapeutic peptides and peptide libraries, M. Ventura, J. Pharm. Biomed. Anal., 2020, 185, 113227
Related Resources




