Welcome Back!
Pressure is a characteristic of the chromatographic conditions employed for the separation. It can be used as a diagnostic tool to understand causes of common issues, thus it is imperative to record the pressure trace.
In LabSolutions, you can display the pressure via a right-click in the chromatogram then select "Display Setting" – "Status". Even if it is not displayed, the pressure is always recorded with every data file and can be displayed during the data processing and evaluation.
The causes of pressure disruptions are varied. They can either be caused by the hardware, by contamination of the eluents, particles in the samples, crystallised buffer, or similar contaminants that clog the system. Even small contaminants can lead to a slow but steady increase in pressure. As already mentioned in Unit 2, temperature also plays a major role. If the temperature rises, the viscosity of the eluent decreases and consequently the pressure also decreases.
However, there are also pressure fluctuations that are completely normal and do not cause concern. For example, if a gradient is run, we actively mix two or more liquids of different densities and viscosities. The result is a uniform increase or decrease in pressure, which in most cases corresponds to the shifted course of the gradient.
This section of the course will investigate the most common pressure issues including fluctuations during the run, and unexpected increases or decreases in pressure.
1. Pressure Fluctuations in LCMS
Pressure fluctuations can have many different causes. The back pressure of an LCMS system is similar to a heartbeat: any irregularity is immediately visible in both the pressure trace and the MS signal (total ion chromatogram - TIC). Therefore, it is crucial to monitor the pressure profile during all runs and investigate abnormalities promptly. The TIC is the total signal intensity for all ions which are detected in a given timeframe.
In LCMS, pressure issues are more critical than in standalone LC because they can also disrupt ionisation stability and vacuum performance in the mass spectrometer.
A. Air Bubbles
Air bubbles in the solvent lines or pump heads are a frequent cause of unstable pressure and can also destabilize the electrospray ionisation (ESI) spray.
Solutions:
- Purge solvent lines thoroughly; use a vacuum syringe on purge valves if necessary.
- Ensure degasser is functioning properly.
- Use low surface tension solvents (e.g., methanol) to help remove stubborn bubbles.
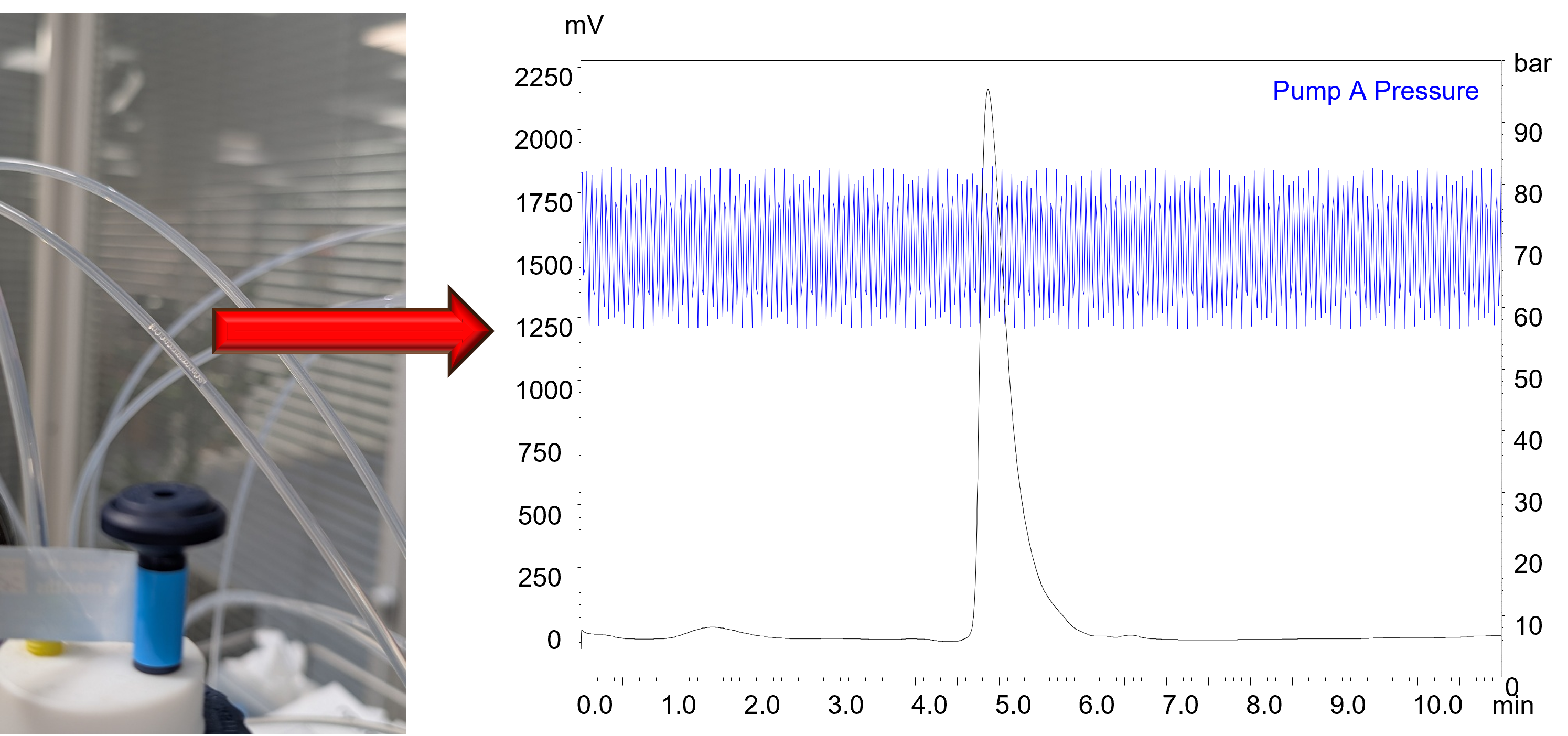
Fig. 1 A characteristic ripple caused by an air bubble, which can impact on the retentivity and robustness of an analysis.
B: Worn Pump Seals or Faulty Check Valves
These can cause sawtooth pressure profiles and unstable spray signals in MS.
Solutions:
- Replace pump seals if pressure drops or leakage is visible around pump heads.
- Clean or sonicate check valves in warm isopropanol. If issues persist, replace them.
C: Leaks at LCMS Interface or Fittings
Leaks not only lower LC backpressure but may also compromise the MS vacuum, resulting in spray instability or vacuum errors.
Solutions:
- Inspect all high-pressure fittings (especially near the column outlet and MS source) for solvent residue or hissing sounds.
- Tighten fittings appropriately or replace ferrules if worn.
- For persistent vacuum instability, check MS source housing for solvent leaks.
D: Contamination or Blockages (LC Side or MS Interface)
Particles, precipitated buffers, or matrix deposits can increase backpressure or partially block flow into the MS.
Solutions:
- Column or guard column blockages. Replace or backflush (if permitted by the column manufacturer).
- ESI probe, nebuliser, or capillary contamination: Clean or replace to restore proper spray and flow.
E: Mobile Phase Issues
Non-volatile buffers (e.g., phosphate) or expired solvents can precipitate and cause clogging, especially at the MS interface.
Solutions:
- Use only LCMS grade volatile buffers (e.g., ammonium formate, ammonium acetate).
- Discard aged solvents and prepare fresh mobile phases daily.
- Filter all mobile phases and ensure proper degassing.

Fig. 2 A contaminated ion source with a non-volatile phosphate-based mobile phase, which can cause pressure issues, as well as sensitivity problems.
2. High Backpressures
Too high pressures are usually due to the column or a blockage. A clogged inline filter of the pump can also cause high pressures. The best way to find the cause is to loosen the first connection at the pump and successively add the components. If the pressure rises sharply, the culprit is identified. Common causes of high back pressure are:
| If the flow rate is incorrectly set too high in the method, this will cause the pressure to increase and cause peaks to elute earlier than expected |
Check the flow rate settings to ensure the velocity is correct. Amend as necessary
|
| A column can become blocked with particulates, especially with dirty samples. This will build on the head of the column and cause the pressure to increase over the course of a batch |
If possible or recommended by the column manufacturer, the column might be backflushed to remove the particulates. However, the build up will occur again if the samples are not properly prepared (i.e. filtering / precipitation etc)
The most likely course of action to correct for a blocked column is to replace the column
Replace the blocked guard column
|
| Immiscible solvents or certain buffers with too high organic modifier might precipitate, which can cause the pressure to increase in the system |
Use the correct mobile phase with compatible solvents
Flush the column out with a cleaning solvent. Ensure the column is fully equilibrated before first use.
|
| The pressure is a function of the column dimensions and particle size. If the incorrect column was installed in the system, it could have a different pressure. It should be noted that pressure may differ between columns of the same dimension and particle morphology but it should be within a reasonable tolerance based on usage. |
Install the correct column dimensions and particle geometry
|
| Air bubbles in the solvent lines or pump heads are a frequent cause of unstable pressure and can also destabilise the ESI spray. |
Purge solvent lines thoroughly; use a vacuum syringe on purge valves if necessary
Ensure degasser is function
Use low surface tension solvents (e.g. methanol) to help remove stubborn bubbles
|
3. Low Backpressures
Low pressure may be caused by:
|
Check the method settings are correct
Check the flow rate accuracy using a calibrated flow rate meter. Alternatively, collect a specific volume and monitor the time it takes to acquire.
|
|
Check for leaks and reconnect the affected part
Replace worn out or damaged pump seals
|
|
Purge all the lines to remove the bubbles
Purge the system with IPA and ensure the check valves are working correctly. Sonicate the check valves if necessary in IPA
|
|
Ensure the flow path is correctly installed
Document the setup of the system for future users to know the configuration
|
|
Check the method for the correct temperature and solvent lines as this can impact on the pressure
Check the correct column is selected if using a column selection valve
|
Key Takeaway
In LCMS, pressure fluctuations affect not only chromatographic performance but also MS spray stability and sensitivity. By combining LC troubleshooting steps with MS-specific checks (leaks, interface contamination, spray monitoring), you can resolve most issues efficiently.
Things to consider daily:
-
- Use LCMS grade solvents and buffers
- Filter at high salt concentration
- After the instrument has finished, the buffer should be flushed out of the system to prevent precipitation issues
- Flush the LCMS with a cleaning solvent mix (i.e. water/acetonitrile (50:50 v/v) at the end of each day to prevent buildup in the source
- Consume limited shelf-life eluents within the shortest time
-
- Filter or centrifuge the samples to remove particulates
- If necessary, also perform purification steps, such as protein precipitation.
- Verify compatibility with the eluents
- Flush the injector if samples with high salt concentration were used.
-
- Monitor both LC backpressure and MS vacuum pressure during runs.
- Inspect and clean the ionisation source and capillary regularly
- Keep the temperature constant to avoid pressure fluctuations
- Keep common consumables in the lab to minimise any potential downtime
- Always display the pressure profile. You will learn a lot about the condition of your system. With a little experience, you will be able to recognise the error based on the pressure.

Fig. 3 Recommended LCMS solvents for Shimadzu Instrumentation.
In the next course unit, we will look at possible causes for missing peaks, ranging from the sample, through the LC and then on to the detector, with simple solutions to solve the problem.
Your Shimadzu LCMS Team
Next unit >>
Related Resources
-
-
Watch short videos explaining analytical intelligence features of Shimadzu HPLC systems
-