Poor Peak Resolution
GC Troubleshooting Course
9 - Poor Peak Resolution
10 - Split Peaks
11 - Response Variability
12 - Retention Time Variability
13 - Course Summary & Test
Poor Peak Resolution
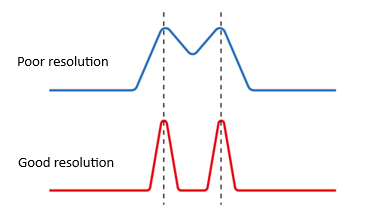
Peak resolution is critical to accurate quantitation and compound identification. Poorly resolved peaks can result in overlapping signals, misidentification, and incorrect integration — particularly when using detectors like FID or TCD that lack mass-selective capabilities.
What is Poor Resolution? Two peaks are considered poorly resolved when:
- They are not separated to baseline.
- The valley between them is shallow or non-existent.
- Integration becomes ambiguous, especially for minor components adjacent to major peaks.
Common Causes & Fixes
1. Non-selective or Suboptimal Column
Cause: The stationary phase lacks selectivity for your target compounds, or the column dimensions are not optimal for your separation.
Fixes:
- Choose a column with a stationary phase tailored for your analytes. This is particularly important for: Isomers (e.g., xylenes, PAHs) and sulphur compounds, which often require special deactivation or selectivity.
- Adjust column dimensions: Longer columns improve resolution (at the cost of runtime); narrower internal diameters provide sharper peaks; use application-specific phases when available.
Application notes and chromatographic literature can be invaluable for selecting appropriate column chemistries.
2. Non-Optimised GC Method Parameters
Cause: The carrier gas velocity or oven temperature ramp is not well matched to the analyte volatility and separation needs.
Fixes:
- Reduce carrier gas linear velocity to increase interaction time with the stationary phase.
- Modify oven program: Use a shallower ramp or isothermal hold in the region where compounds are co-eluting; lower the initial temperature for better focusing.
Slowe ramps and lower flows increase resolution but can also increase runtime.
3. Column Overload
Cause: Injecting too much sample causes peak broadening and overlap, particularly when small peaks elute near large ones.
Fixes:
- Dilute the sample or increase the split ratio.
- Ensure injection volumes are appropriate for the column dimensions and film thickness.
Be cautious when small peaks are masked by overloaded larger peaks — reducing the injection volume may improve both visibility and resolution but will reduce sensitivity.
4. Challenging Analyte Chemistry
Some compounds are notoriously difficult to separate due to:
- Structural similarity (e.g., isomers like ethylbenzene and xylenes).
- Adsorption issues (e.g., sulphur compounds).
- Close boiling points or high polarity.
Fixes:
- Use specialist columns (e.g., PAH-selective).
- Use deactivated components in the sample flow path (e.g. Sulfinert fittings).
- Consider derivatisation during sample prep to improve chromatographic behaviour.
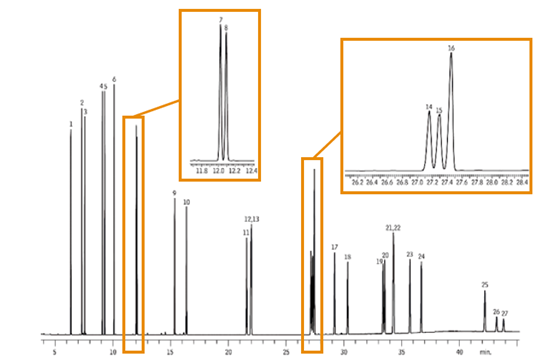
Case Example - Chromatogram Comparison:
A common column phase fails to separate Benzo[b]fluoranthene, Benzo[k]fluoranthene, and Benzo[j]fluoranthene, which are successfully resolved using a PAH-specific column.
This highlights the impact of selecting the right stationary phase — a small change in chemistry can dramatically improve separation.
Related Resources





